

Label each glass with the emulsifier that was added, and label the empty glass “control.” Label the data sheet with the emulsifiers you will be testing.
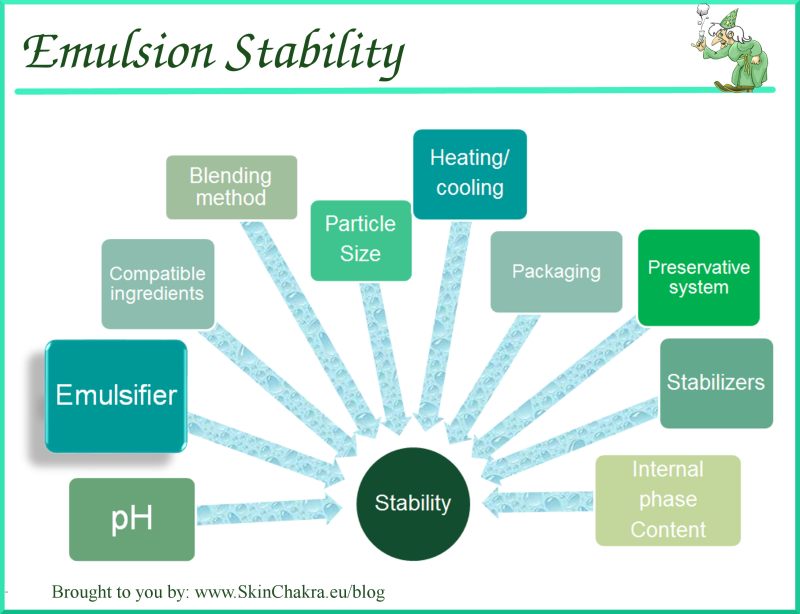
For instance, after adding an effective emulsifier to oil and vinegar and mixing thoroughly, separation of the oil from the vinegar will take much longer or won’t happen at all. They contain both hydrophobic and hydrophilic regions and are able to attract and “hold hands” with polar and non-polar molecules simultaneously, pulling them together to form a special type of mixture called an emulsion. Emulsifiers are the hand-holders of the molecule world. How can we bring together polar and non-polar molecules to make something delicious like mayonnaise (which is essentially a combination of water and oil) or salad dressing? We need an emulsifier. Because oils also repel water, they are called hydrophobic, which means “water-fearing.” The carbon (black) and hydrogen (white) in this non-polar fatty acid molecule share electrons evenly and are neither negatively or positively charged. Oils repel polar molecules such as those found in vinegar. You can observe this phenomenon by placing a few drops of oil on the surface of a bowl of water-eventually the drops will form a single large oil slick. Most of the atoms in a fatty acid molecule share electrons evenly and are neither negatively nor positively charged (although fatty acids do contain small regions of polarity-just not enough to make the whole molecule polar.) Non-polar molecules love other non-polar molecules and will glom together when mixed with water. Fats and oils are composed primarily of long molecules called fatty acids (usually bound together by glycerol molecules into groups of three called triglycerides). Oils are a type of fat (like butter, shortening, and lard) and are considered non-polar. Polar molecules are attracted to water molecules-which are also polar-and are called hydrophilic, which means “water loving.” Polar molecules are generally attracted to other polar molecules because their slightly negative poles have an affinity for their slightly positive poles.

These slightly charged poles arise because one or more atoms in the molecule are electronegative, meaning that they tug electrons-which are negatively charged-towards them, creating an uneven distribution of charge within the molecule. Water, acetic acid, and alcohol are all examples of polar molecules-molecules that have a slightly negative charge at one end, or pole, and a slightly positive charge at another end. Most vinegars are solutions of acetic acid and water (plus some other acids and alcohols, depending on the type of vinegar you are using). The electronegative oxygen in a water molecule pulls electrons away from the two hydrogens, creating an uneven distribution of charge within the molecule. This happens because vinegar and oil are made of very different types of molecules that are attracted to their own kind. No matter how hard you try to shake, stir, or whisk oil and vinegar together, they eventually separate. If you’ve ever tried to make salad dressing from scratch, you know that one of the biggest challenges is getting the oil and the vinegar to mix properly.


 0 kommentar(er)
0 kommentar(er)
